Services
- Health Economics
- Systematic Literature Review
- Meta-analysis
- Medical Writing
- Data and Dashboards
Health Economic Modeling and Analysis
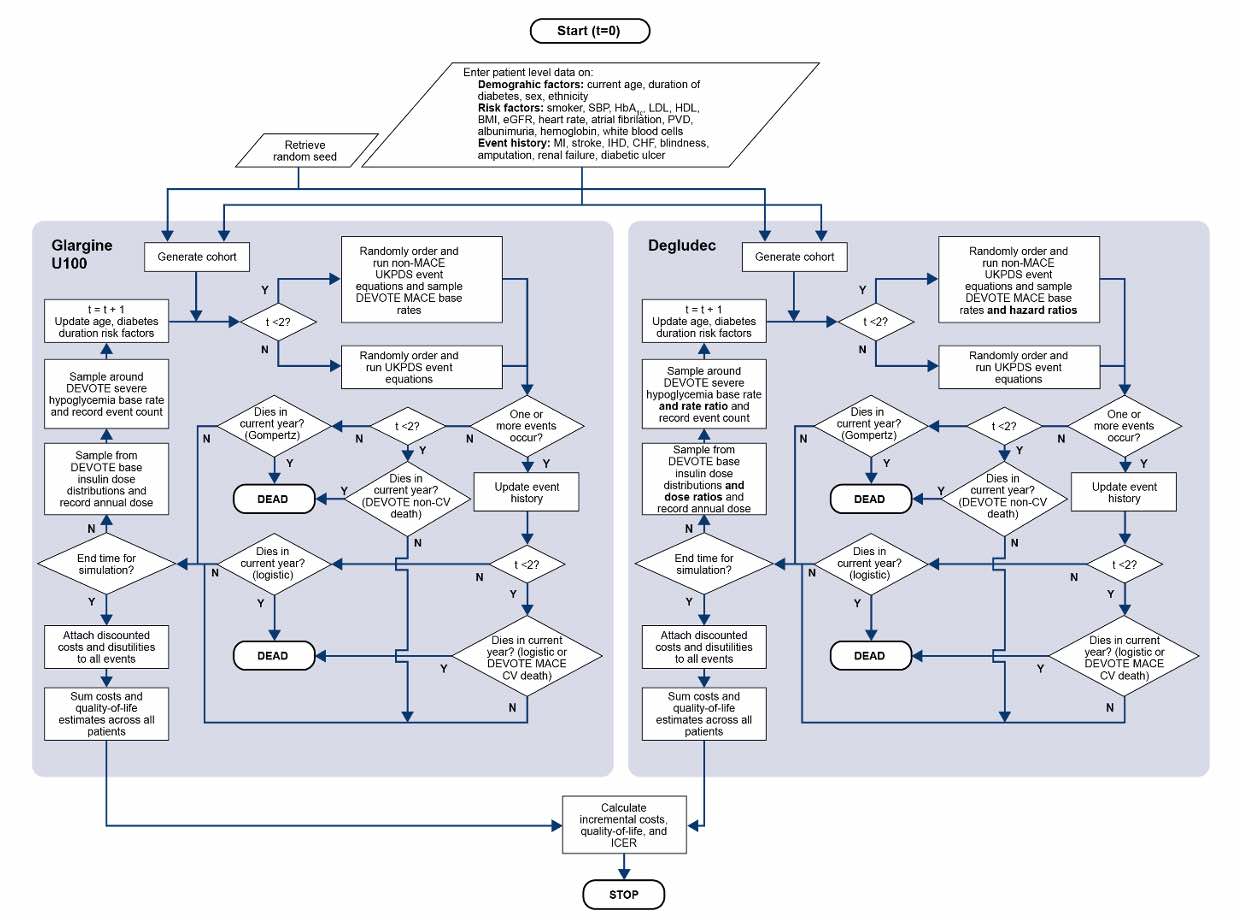
Covalence has a wealth of health economic modeling expertise, spanning numerous therapy areas, modeling objectives, model structures, and technology platforms.
Furthermore, in the reimbursement context, Covalence has consistently delivered models that have resulted in successful reimbursement of the health technology of interest, including models that have formed the basis of successful submissions to the National Institute for Health and Care Excellence (NICE), the Scottish Medicines Consortium (SMC), and Canada's Drug Agency (the CDA-AMC, formerly CADTH).
Systematic Literature Review
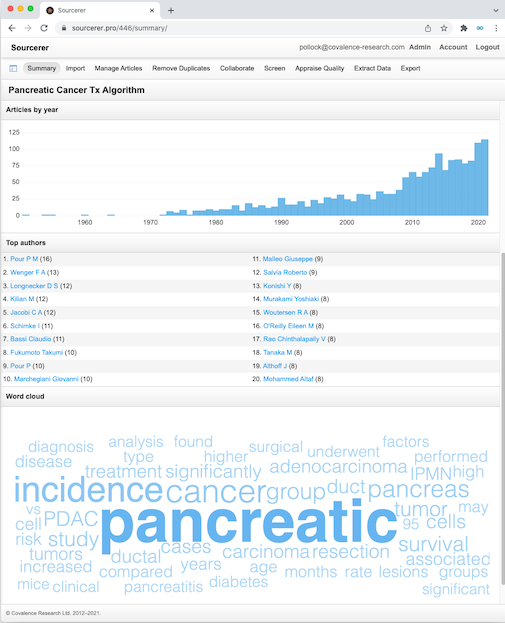
The Covalence team has performed dozens of systematic literature reviews, including reviews that have formed the basis of widely-cited peer-reviewed manuscripts and reimbursement submissions.
Since systematic literature reviews often form the bedrock of evidence synthesis efforts such as network meta-analyses and evidence generation efforts such as cost-utility modeling, Covalence takes the process very seriously. So seriously, in fact, that we've developed our own in-house software for conducting systematic literature reviews: Sourcerer. Sourcerer allows Covalence to rapidly screen large volumes of literature and provide bibliographies and literature review outputs in a consistent format.
Meta-analysis
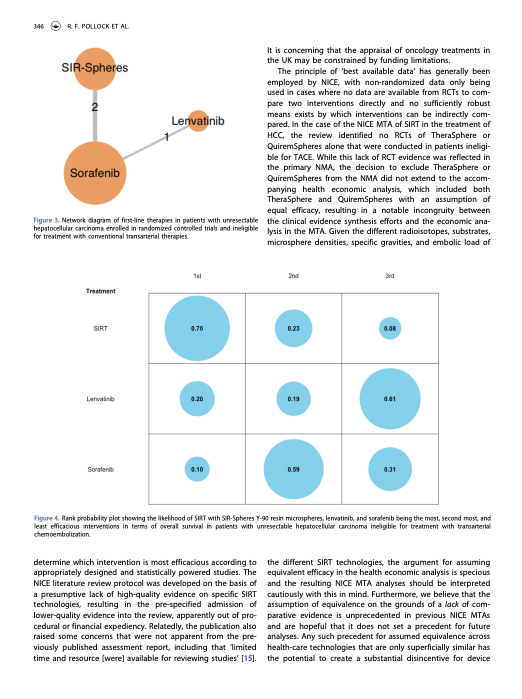
Covalence has extensive experience running meta-analyses, including everything from pooling of data from multiple trials of the same interventions, through indirect treatment comparisons, to complex network meta-analyses.
In the interests of ensuring that meta-analyses are conducted and reported in accordance with best practice guidelines from ISPOR and NICE, Covalence sponsors the BUGSnet project and works in close collaboration with the University of Waterloo to steward its ongoing development.
Medical Writing

Medical writing and value communications are among the core services offered by Covalence. This includes the preparation of systematic literature reviews, global value dossiers and value messages to support reimbursement as well as landscape reviews, manuscripts, posters, slide kits and medical education resources.
Attention to detail and a desire to produce the highest quality deliverables possible is at the cornerstone of everything we do. We work closely with clients to ensure that each project is tailored to meet the specific needs of the market and therapy area in question ensuring that all deliverables are customized and underline the nuances and value of each individual intervention.
The Covalence team have a wealth of experience in medical writing across a broad range of therapy areas with particular expertise in diabetes, oncology and virology. Examples of recent manuscripts published by the Covalence team can be found here.
Data and Dashboard Development
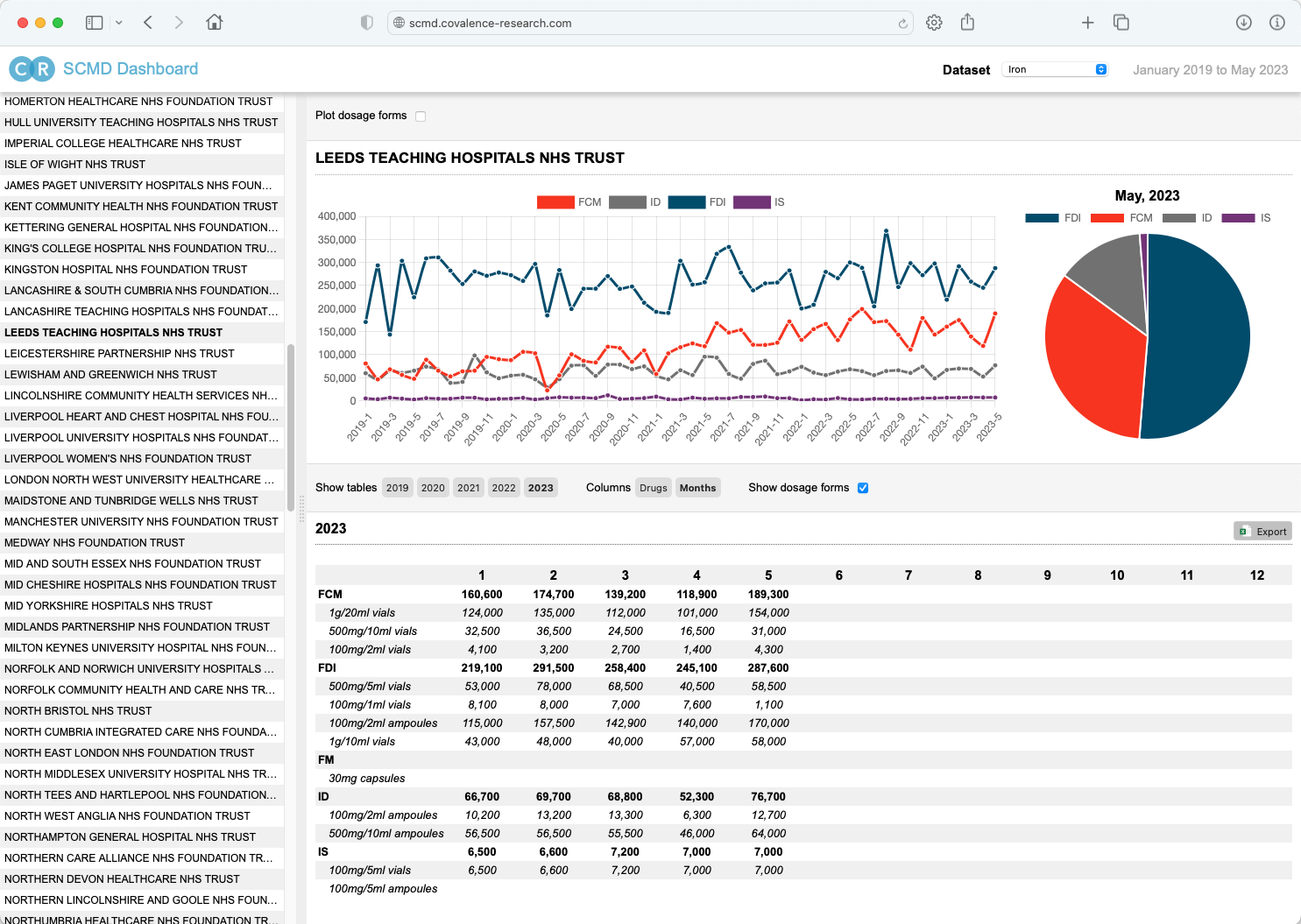
Data underpins everything we do at Covalence and, whether it's clinical or commercial, it's important to able to quickly gain insights into key signals and messages from the data.
So whether it's one of our off-the-shelf offerings such as our Secondary Care Medicines Data (SCMD) Dashboard (right) or a bespoke dashboard to unearth themes or trends from your own datasets, we are well equipped to deliver elegant and incisive dashboards using an array of modern, secure, and efficient technologies, including Shiny and Angular.
For enquiries pertaining to Covalence's SCMD Dashboard, please contact scmd-enquiries@covalence-research.com.
About Us
 Richard Pollock MA MSciHealth Economist, UK
Richard Pollock MA MSciHealth Economist, UK
Richard is a Health Economist and Director at Covalence.
Having graduated with a first-class MSci in Natural Sciences from the University of Cambridge in 2007, he has since worked as a health economist for over 18 years. He has developed models, formulated analysis plans, and managed health technology assessment submissions that have contributed to successful reimbursement decisions at the SMC, NICE, AMCP and CADTH, and has published over 100 peer-reviewed publications and hundreds of congress and conference presentations.
 Johannes Pöhlmann BA MSc MPHSenior Health Economist, Germany
Johannes Pöhlmann BA MSc MPHSenior Health Economist, Germany

Johannes is a Senior Health Economist at Covalence.
Johannes graduated with a BA in Political Science and Economics from the University of Munich in 2013. He went on to complete an MSc in Demography & Health at the London School of Hygiene and Tropical Medicine in 2015 and an MPH from the University of Munich in 2016. He has since worked on health economic evaluations of public health interventions and health technology assessments, including for biosimilar infliximab in Switzerland, at the Zurich University of Applied Sciences (Switzerland) and on autonomic dysfunction in heart failure at the University of Mainz (Germany).
 Jayne Smith-Palmer DPhilSenior Consultant, UK
Jayne Smith-Palmer DPhilSenior Consultant, UK
Jayne is a Senior Consultant at Covalence, specialising in medical writing, evidence synthesis, and value communication.
Jayne graduated with a BSc in Biology from the University of St Andrews in 2002. She went on to complete a DPhil in biophysics at Oxford University in collaboration with the National Institute for Medical Research, London, UK and the National Heart Lung and Blood Institute, Bethesda, MD, USA. Since then, she has gained over 15 years of experience in medical writing and value communications, and has coauthored over 30 publications in the peer-reviewed literature.
Shaista Nisar PhDSenior Medical Writer, UK

Shaista is a Senior Medical Writer at Covalence.
Shaista graduated with a first-class BSc degree in pharmacology from King’s College London in 2005, and later went on to complete a PhD in pharmacology at the University of Bristol. Shaista subsequently held a post-doctoral position funded by the British Heart Foundation, during which she authored several peer-reviewed publications. Shaista has worked in market access consulting since 2015 and has over 10 years of experience in medical writing, value communication and primary research.
 Neesha Nanu BSc MScSenior Evidence Synthesis Consultant, UK
Neesha Nanu BSc MScSenior Evidence Synthesis Consultant, UK
Neesha is a Senior Evidence Synthesis Consultant at Covalence.
Neesha graduated with a BSc in Biomedical Science from the University of Kent in 2015 and went on to complete an MSc in epidemiology at Imperial College London. She subsequently worked as a Research Assistant at Imperial where she worked on a continuously-updated systematic literature review funded by the World Cancer Research Fund International on the role of diet, nutritional status, anthropometric characteristics and physical activity in cancer prevention and prognosis. Neesha joined Covalence in 2022 where she continues to work on epidemiological research and novel evidence synthesis projects.
Tray Brown BSc MScPrincipal, Czech Republic

Tray is a Principal at Covalence.
Tray previously held senior leadership and technical modelling roles in consulting and academia, and joined Covalence as Principal in 2024. Prior to joining Covalence, Tray was Director of Health Economics at HEOR Ltd where he provided strategic consulting and led the development of models in a wide range of disease areas including cardiovascular, oncology, infectious diseases and autoimmune indications. His experience includes building global models for later country-specific adaptations as well as models for HTA submissions to NICE, interpreting both real-world and clinical trial data to produce complex cost-effectiveness models and conducting value of information analyses. Tray has also developed and advised on early-stage models for strategic planning and budget impact for both global and country-specific settings, including in the US and UK.
 Matt Tucker BA MScHealth Economist, UK
Matt Tucker BA MScHealth Economist, UK
Matt is a Health Economist at Covalence.
Matt graduated with a first-class degree in BA Economics from the University of Leicester in 2019. He then earned a distinction in MSc Health Economics from the University of York in 2020, as part of an NIHR studentship scheme. He went on to join HEOR Ltd, where he gained expertise in early economic evaluations and health technology assessments across several therapeutic areas, including oncology, obesity, immune disorders, and COVID-19, utilising a range of modelling techniques. Matthew joined Covalence in 2024, where he continues his varied work across multiple therapeutic areas and modeling methodologies.
 Martin Field PhDSenior Medical Writer, UK
Martin Field PhDSenior Medical Writer, UK

Martin is a Senior Medical Writer at Covalence.
Martin graduated from the University of Cambridge with a BA and MSci in Natural Sciences in 2014 and then completed a PhD in neuropharmacology at University College London in 2018. He subsequently gained three years of postdoctoral research experience at the University of Oxford where he collaborated with consultant neurosurgeons to study drug action in the human brain. Martin then began his career in medical writing at Clarivate where he worked on a range of deliverables and therapeutic areas for clients across the pharmaceutical industry before joining Covalence in 2025.
 Rose Hart PhDSenior Health Economist, UK
Rose Hart PhDSenior Health Economist, UK

Rose is a Senior Health Economist at Covalence.
Rose completed her BSc and PhD in Biomedical Science from the University of Sheffield, which included industry experience as a BioPharm researcher at GSK. In 2017, she transitioned into health economics at Lumanity (formerly BresMed). During this period, Rose developed expertise in non-standard modelling and became proficient at building models in Excel, R and R-Shiny, leading the expansion of their R-modelling service offering. An active thought leader, she delivers an annual course on R modelling at ISPOR EU. Joining Covalence in 2025 as a Senior Health Economist, Rose now focuses on creating bespoke modelling solutions to demonstrate therapeutic value, driven by interests in data visualization, scalable solutions, and advanced analytics.
Publications
2025
- Alshannaq H, Matuoka J, , , Lynch P, Norman GJ. Real-time continuous glucose monitoring vs self-monitoring of blood glucose in distinct multi-ethnic cohorts of patients living with insulin-treated type 2 diabetes in the United States: A cost-utility analysis from a Medicare perspective. J Manag Care Spec Pharm. 2025;31(8):752-763.
- De la Torre-Aláez M, Matilla A, Varela M, Iñarrairaegui M, Reig M, Lledó JL, Arenas JI, Lorente S, Testillano M, Márquez L, Iserte G, Argemí J, Gómez-Martin C, Rodríguez-Fraile M, Bilbao J, , , Agirrezabal I, Sangro B. Health-related Quality of Life in Patients with Unresectable Hepatocellular Carcinoma Treated with SIRT and Nivolumab: a Sub-analysis of the NASIR-HCC Trial. J Patient Rep Outcomes. 2025;9(1):39.
- Detlie TE, Karlsen LN, Jørgensen E, , . Evaluating the Cost-utility of Ferric Derisomaltose versus Ferric Carboxymaltose in Patients with Inflammatory Bowel Disease and Iron Deficiency Anaemia in Norway. J Med Econ. 2025;28:291–301.
- Hanaire H, Ozdemir Saltik AZ, , , Sambuc C, Grangeon A, De Portu S, Koch P, Cohen O, Thivolet C. Cost-Utility Analysis of the MiniMed™ 780G Advanced Hybrid Closed-Loop System Versus Intermittently Scanned Continuous Glucose Monitoring with Multiple Daily Insulin Injections in People with Type 1 Diabetes in France. Diabetes Technol Ther. 2025. In press.
- Kim JY, Ilham S, Alshannaq H, , , Norman GJ, Jin S-M, Kim JH. Cost-effectiveness analysis of Real-time Continuous Glucose Monitoring versus Self-monitoring of Blood Glucose in People with Type 2 Diabetes Treated with Non-intensive Insulin Therapy in South Korea. J Diabetes Sci Technol. 2025. In press.
- Lindgren S, Strid H, Hjortswang H, Manxhuka B, , . A Swedish Cost-utility Analysis of Ferric Derisomaltose versus Ferric Carboxymaltose in the Treatment of Iron Deficiency Anemia in Patients with Inflammatory Bowel Disease. J Med Econ. 2025;28(1):567-575.
- , Joecker A, Wittki T, , , Chase J. Point of Care Nucleic Acid Testing for Influenza-Like Illness: A Cost–Consequence Analysis for High-Risk Patients in Primary Care in Germany. Adv Ther. 2025;42(5):2385-2402.
- , , Serné EH, Tuomaala A-K, Jendle J, Choudhary P, Bosi E, Mohan V, van den Heuvel T, Cohen O, . A Systematic Literature Review and Meta-analysis of Real-World Evidence on Commercially Available Automated Insulin Delivery Systems in People with Type 1 Diabetes. Diabetes Obes Metab. 2025. In press.
- Serné EH, Buompensiere MI, de Portu S, , , Cohen O. Automated insulin delivery versus standard of care in the management of people living with type 1 diabetes and HbA1c <8%: a cost-utility analysis in The Netherlands. Diabetes Technol Ther. 2025;27(8):631-640.
- Sufyan H, Ozdemir Saltik AZ, Yu JJ, de Portu S, , , Cohen O. Improving Time-in-Range in Type 1 Diabetes: Projecting the Clinical and Cost Implications of Automated Insulin Delivery. Diabetes Technol Ther. 2025. In press.
- Younossi ZM, Paik JM, Henry L, , Stepanova M, Nader F. Economic evaluation of non-invasive test pathways for high-risk metabolic dysfunction-associated steatotic liver disease (MASLD) in the United Kingdom (UK). Ann Hepatol. 2025;30(2):101789.
2024
- Agirrezabal I, , Carion PL, Shergill S, VK Brennan, Pereira H, Chatellier G, Vilgrain V. Association of Adverse Events and Quality of Life in Patients with Unresectable Hepatocellular Carcinoma. Qual Life Res. 2024;33:3377–3386.
- Alshannaq H, , Joubert M, , Norman GJ, Lynch PM, Roze S. Cost-utility of real-time continuous glucose monitoring versus self-monitoring of blood glucose in people with insulin-treated type 2 diabetes in France. J Comp Eff Res. 2024;13(3):e230174.
- Bjorner JB, Kennedy N, Lindgren S, . Hypophosphatemia attenuates improvements in vitality after intravenous iron treatment in patients with inflammatory bowel disease. Qual Life Res. 2024;33(8):2285-2294.
- Cardell L, Sterner T, , Slættanes AK, Svärd M, . Modelling the impact of sublingual immunotherapy versus subcutaneous immunotherapy on patient travel time and CO2 emissions in Sweden. Sci Rep. 2024;14(1):1575.
- Cardell L, Sterner T, , Slættanes AK, Svärd M, . Modelling the Costs of Sublingual Immunotherapy versus Subcutaneous Immunotherapy Based on Clinical Appointments and Impacts of Patient Travel in Sweden. ClinicoEcon Outcomes Res. 2024;16:493-506.
- Hadzi Boskovic D, , , , Hwang S, Bruhn B. Systematic Literature Review of Studies Reporting Measures of Functional Outcome or Quality of Life in People with Negative Symptoms of Schizophrenia. Patient Relat Outcome Meas. 2024;15:199-217.
- Iqbal TH, Kennedy N, Dhar A, , . Cost-utility analysis of ferric derisomaltose versus ferric carboxymaltose in patients with inflammatory bowel disease and iron deficiency anemia in England. J Med Econ. 2024;27(1):392—403.
- Kim JY, Ilham S, Alshannaq H, , , Norman GJ, Jin S-M, Kim JH. Real-time Continuous Glucose Monitoring vs. Self-monitoring of Blood Glucose: Cost-utility in South Korean Type 2 Diabetes Patients on Intensive Insulin. J Med Econ. 2024;27:1245–1252.
- Mathieu C, , Gillard P, Cohen O, Vigersky R, de Portu S, Ozdemir Saltik AZ. The health economics of automated insulin delivery systems and the potential use of time in range in diabetes modeling: a narrative review. Diabetes Technol Ther. 2024;26(S3):66—75.
- Merino-Torres JF, Ilham S, Alshannaq H, , , Norman GJ. Cost-utility of real-time continuous glucose monitoring versus self-monitoring of blood glucose in people with insulin-treated type 2 diabetes in Spain. ClinicoEcon Outcomes Res. 2024;16:785–7
- , Weller M, Marcellusi A, Grabe-Heyne K, Krott-Coi L, , . High costs, low quality of life, reduced survival, and room for improving treatment: an analysis of burden and unmet needs in glioma. Front Oncol. 2024;14:1368606.
- Zhang F, Shen A, , . A Cost-utility Analysis of Ferric Derisomaltose versus Ferric Carboxymaltose in Patients with Iron Deficiency Anemia in China. Adv Ther. 2024;41:4191–4204.
- Zhao T, Tew M, Feenstra T, van Baal P, Willis M, Valentine WJ, Clarke PM, Hunt B, Altunkaya J, Tran-Duy A, , Malkin SJP, Nilsson A, McEwan P, Foos V, Leal J, Huang ES, Laiteerapong N, Lamotte M, Smolen H, Quan J, Martins L, Ramos M, Palmer AJ. The Impact of Unrelated Future Medical Costs on Economic Evaluation Outcomes for Different Models of Diabetes. Appl Health Econ Health Policy. 2024;22:861–869.
2023
- Alshannaq H, Cogswell G, , , Norman GJ, Lynch P, Roze S. Cost-utility of real-time continuous glucose monitoring versus self-monitoring of blood glucose and intermittently-scanned continuous glucose monitoring in people with type 1 diabetes receiving multiple daily insulin injections in Denmark. Diabetes Obes Metab. 2023;25(9):2704—2713.
- Alshannaq H, Isitt JJ, , Norman GJ, Cogswell G, Lynch PM, Roze S. Cost-utility of real-time continuous glucose monitoring versus self-monitoring of blood glucose in people with insulin-treated type 2 diabetes in Canada. J Comp Eff Res. 2023;12(10):e230075.
- Fukumoto S, Murata T, Osuga Y, . Incidence of hypophosphatemia after administration of intravenous iron: a matching-adjusted indirect comparison of data from Japanese randomized controlled trials. Adv Ther. 2023;40(11):4877—4888.
- Grabe-Heyne K, Henne C, Mariappan P, Geiges G, , . Intermediate and high-risk non-muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs. Front Oncol. 2023:13:1170124.
- Grabe-Heyne K, Henne C, Odeyemi I, , , . Evaluating the cost-utility of intravesical Bacillus Calmette-Guérin versus radical cystectomy in patients with high-risk non-muscle-invasive bladder cancer in the UK. J Med Econ. 2023;26(1):411—421.
- Hu S, Wu D, Wu J, Zhang Y, Bøgelund M, , . Disutilities associated with intravenous iron infusions: results from a time trade-off survey and diminishing marginal utility model for treatment attributes in China. Patient Relat Outcome Meas. 2023;14:253-267.
- Jendle J, Buompensiere MI, Ozdemir Saltik AZ, de Portu S, , , Cohen O. A European cost-utility analysis of the MiniMed™ 780G advanced hybrid closed-loop system versus intermittently scanned continuous glucose monitoring with multiple daily insulin injections in people living with type 1 diabetes. Diabetes Technol Ther. 2023;25(12):864—876.
- Kennedy NA, Achebe MM, Biggar P,, . A systematic literature review and meta-analysis of the incidence of serious or severe hypersensitivity reactions after administration of ferric derisomaltose or ferric carboxymaltose. Int J Clin Pharm. 2023;45(3):604—612
- , Shergill S, Carion PL, von Oppen N, Agirrezabal I, Brennan VK. Advances in delivery of selective internal radiation therapy (SIRT): economic and logistical effects of same-stay work-up and procedure in the treatment of unresectable liver tumors in England. Adv Ther. 2023;40(1):294—309.
- , Slættanes AK, Brandi H, Grand TS. A Cost-utility Analysis of SQ® Tree SLIT-tablet versus placebo in the treatment of birch pollen allergic rhinitis from a Swedish societal perspective. Clinicoecon Outcomes Res. 2023;15:69—86.
- Valentine WJ, Hoog M, Mody R, Belger M, . Long-term cost-effectiveness analysis of tirzepatide versus semaglutide 1.0 mg for the management of type 2 diabetes in the US. Diabetes Obes Metab. 2023. 25(5):1292—1300.
2022
- Hu S, Liu L, , , Wu D, Zhang Y. Intravenous iron for the treatment of iron deficiency anemia in China: a patient-level simulation model and cost-utility analysis comparing ferric derisomaltose with iron sucrose. J Med Econ. 2022;25(1):561-570.
- , Bergenheim K, Sanchez J-JG, Rao N, Briggs A, . Modeling chronic kidney disease in type 2 diabetes mellitus: a systematic literature review of models, data sources, and derivation cohorts. Diabetes Ther. 2022;13(4):651-677.
- , Dhar A, Johnson M. Economic analysis of intravenous iron in patients with iron deficiency anemia due to inflammatory bowel disease: considerations for clinicians [letter]. Clinicoecon Outcomes Res. 2022;14:163-165.
- , Kalra PA, Kalra PR, Ahmed FZ. A systematic review, meta-analysis, and indirect comparison of blindly adjudicated cardiovascular event incidence with ferric derisomaltose, ferric carboxymaltose, and iron sucrose. Adv Ther. 2022;39(10):4678-4691.
- , Norrbacka K, Boye KS, Osumili B, Valentine WJ. The PRIME type 2 diabetes model: a novel, patient-level model for estimating long-term clinical and cost outcomes in patients with type 2 diabetes mellitus. J Med Econ. 2022;25(1):393-402.
- Rønborg S, Grand TS, Brandi H, . ITULAZAX® versus Alutard SQ® in the treatment of allergic rhinitis induced by pollen from the birch homologous group: a cost-minimization modeling analysis from the Danish societal perspective. Clin Transl Allergy. 2022;12(11):e12196.
2021
- Felizzi F, Paracha N, , Ray J. Mixture cure models in oncology: a tutorial and practical guidance. Pharmacoecon Open. 2021;5(2):143–155.
- , Brennan VK, Shergill S, Colaone F. A systematic literature review and network meta-analysis of first-line treatments for unresectable hepatocellular carcinoma based on data from randomized controlled trials. Expert Rev Anticancer Ther. 2021;21(3):341–349.
- , Colaone F, Shergill S, Brennan VK, Agirrezabal I. Effects of trial population selection on quality of life and healthcare decision-making: a systematic review and example in the treatment of hepatocellular carcinoma with radioembolization. Clinicoecon Outcomes Res. 2021;13:835–841.
- , Muduma G. An economic analysis of ferric derisomaltose versus ferric carboxymaltose in the treatment of iron deficiency anemia in patients with inflammatory bowel disease in Norway, Sweden, and Finland. Clinicoecon Outcomes Res. 2021;13:9–18.
2020
- Brennan VK, Colaone F, Shergill S, . A cost-utility analysis of SIR-Spheres Y-90 resin microspheres versus best supportive care in the treatment of unresectable metastatic colorectal cancer refractory to chemotherapy in the UK. J Med Econ. 2020;23(12):1588-1597.
- Igarashi A, Hunt B, Wilkinson L, Langer J, . Lower drug cost of successfully treating patients with type 2 diabetes to targets with once-weekly semaglutide versus once-weekly dulaglutide in Japan: a short-term cost-effectiveness analysis. Adv Ther. 2020;37(10):4446-4457.
- Loughnane F, Muduma G, . Development of a resource impact model for clinics treating pre-operative iron deficiency anemia in Ireland. Adv Ther. 2020;37(3):1218-1232.
- , Biggar P. Indirect methods of comparison of the safety of ferric derisomaltose, iron sucrose and ferric carboxymaltose in the treatment of iron deficiency anemia. Expert Rev Hematol. 2020;13(2):187-195.
- , Brennan VK, Peters R, Paprottka PM. Association between objective response rate and overall survival in metastatic neuroendocrine tumors treated with radioembolization: a systematic literature review and regression analysis. Expert Rev Anticancer Ther. 2020;20(11):997-1009.
- , Colaone F, Guardiola L, Shergill S, Brennan VK. A cost analysis of SIR-Spheres yttrium-90 resin microspheres versus tyrosine kinase inhibitors in the treatment of unresectable hepatocellular carcinoma in France, Italy, Spain and the UK. J Med Econ. 2020;23(6):593-602.
- , Muduma G. A patient-level cost-effectiveness analysis of iron isomaltoside versus ferric carboxymaltose for the treatment of iron deficiency anemia in the United Kingdom. J Med Econ. 2020;23(7):751-759.
- , Cerri K, Sbarigia U, Chan EKH, , Valentine WJ, Bonroy K. Impact of stigma on people living with chronic hepatitis B. Patient Relat Outcome Meas. 2020;11:95-107.
- Walter T, Hawkins NS, , Colaone F, Shergill S, Ross PJ. Systematic review and network meta-analyses of third-line treatments for metastatic colorectal cancer. J Cancer Res Clin Oncol. 2020;146(10):2575-2587.
Contact
Project-related or other enquiries can be sent to info@covalence-research.com.
Phone
UK
+44 20 8638 6525
Enquiry form
Address
Covalence is headquartered at Rivers Lodge, West Common, Harpenden, AL5 2JD.

